New
for Alzheimer's Disease
Detecting TAU or amyloid alone doesn't mean Alzheimer's
It's the infiltration -depth & specific brain regions affected
that truly matters
Comparison PET scan, MRI scan and Envoy3D for cancer
Iig
Detecting ta risk
In nature the most of chronic diseases are 3D diseases...but we treat these diseases by 2D thinking...
- Current Approach: Many diseases are studied in simplified 2D models, such as cell cultures on flat surfaces or cross-sectional imaging.
- Reality: These diseases involve complex 3D spatial interactions—e.g., cellular communication, tissue microenvironments, and dynamic system-wide processes—that cannot be fully captured in 2D frameworks.
- Result: Treatments based on 2D data may not account for spatial nuances, leading to limited efficacy or unexpected side effects.
Drug Failures in Alzheimer's and Parkinson's:
- For Alzheimer's, amyloid drugs target 2D amyloid plaques but fail to address the 3D interplay of neurons, glia, blood vessels, and metabolic processes.
- For Parkinson's, treatments often focus on neurotransmitter replacement (dopamine) without considering the spatial spread and interaction of alpha-synuclein aggregates.
Cancer as a 3D Example:
- Tumor Microenvironment (TME): Understanding cancer requires mapping not just tumor cells but their 3D interactions with immune cells, blood vessels, and the extracellular matrix.
- Why 2D Thinking Fails: Drugs designed based on isolated cancer cells in 2D environments often fail when tested in real 3D tissues, where spatial constraints and interactions change their behavior.
Neurological Diseases and the Brain:
- The brain operates in 3D networks, with neurons, glia, and vasculature interacting in spatially organized ways. 2D methods like MRI or PET imaging provide only a slice of the whole system.
- Example: ALS involves degeneration of motor neurons in 3D spinal cord tracts. Treatments targeting a single pathway often miss broader spatial dynamics of degeneration.
Rare Diseases:
- Rare 3D diseases like Leigh Syndrome or Sturge-Weber Syndrome are particularly hard to treat because they involve spatially distributed effects in multiple organ systems.
- Why Treatments Fail: A lack of spatially aware models means therapies might focus on isolated symptoms rather than interconnected systems.
The Shift to 3D Thinking:
- Spatial AI and 3D Models: Using technologies like Spatial AI, 3D organoids, and holographic imaging can help map these diseases more accurately.
- Precision Medicine in 3D: Treatments can be designed to target not just the biological pathways but their spatial context—how molecules, cells, and systems interact in 3D.
Transforming Cancer Treatment: Static Data to Dynamic Precision with Envoy3D AI and 3D Spatial Precision Medicine
AI-driven tools can identify under-infiltrated regions, allowing oncologists to adjust treatments that enhance immune cell access to resistant tumor regions.
Envoy3D can be considered a Dynamic Agent in 3D. It operates as a spatially aware AI system, understanding and interacting with complex environments in three dimensions, such as tumor microenvironments (TME) in cancer research. As a Dynamic Agent, Envoy3D adapts its analysis and decision-making in real-time by processing 3D spatial data, allowing it to model and predict the behavior of biological systems or other complex environments more accurately.
In practical terms, Envoy3D can assess the relationships between various entities in a 3D space—like immune cells, blood vessels, and cancer cells—adjusting its approach as it learns from the environment. This adaptability and spatial awareness make it ideal for precision applications, such as guiding immunotherapies or any other context requiring a deep understanding of how 3D systems evolve over time.
Traditional drug development was based largely on 2D models, like cell cultures or simplified assays, which limited the understanding of spatial relationships in complex tissues. Now, with 3D spatial technology, we can explore drug interactions within realistic tissue structures, uncovering new insights into how drugs behave in dynamic environments like tumor microenvironments (TMEs). This shift opens up a new frontier for drug discovery, allowing us to create more effective, precisely targeted treatments that were unimaginable in 2D.
Patent Pending:
i-DEPOT nr: 148645, 148641, 1148633, 148631, 148630, 148637, 148636, 148635, 148634, 148642,148710
System and Method for Accelerating Drug Development, Personalized Dynamic Treatment, and Predictive Prevention Using Digital Twins, Digital Cousins, and Spatial-to-Spatial AI
Multi-Agent AI system for Tumor Microenvironment TME characterization and Dynamic Analysis ID 148763
Name: Guy A Bisschops h.o.d.n. Neuroteg AI
.Common abstraction behind Digital Twins, Digital Cousins, and Spatial-to-Spatial AI
All three of these concepts rely on the idea of representation and simulation, but with different levels of fidelity and focus. They create virtual models that allow us to study, experiment, and interact with complex real-world systems, whether it's for highly detailed replication (Digital Twins), more flexible experimentation (Digital Cousins), or spatial context-awareness (Spatial-to-Spatial AI).
The common abstraction across all three is the modeling of dynamic systems—Digital Twins for precise replication, Digital Cousins for more flexible generalizations, and Spatial-to-Spatial AI for understanding spatial dynamics and relationships. Each serves a unique purpose depending on the level of detail and interaction required.
The Tumor Microenvironment (TME) plays a pivotal role in cancer progression, metastasis, and treatment resistance. The interactions between cancer cells, immune cells, blood vessels, and stromal components within the TME determine how tumors grow, evade the immune system, and respond to therapies. Current methods of cancer treatment often overlook the spatial complexity of the TME, leading to suboptimal treatment outcomes.
Stromal agents can significantly enhance drug delivery by targeting and modifying the stromal components within the Tumor Microenvironment (TME). The dense extracellular matrix (ECM) and cancer-associated fibroblasts (CAFs) that make up the stroma are often major barriers to effective drug penetration, especially in desmoplastic or fibrotic tumors. By focusing on how stromal cells contribute to these physical and biochemical barriers, stromal agents can play a pivotal role in improving the efficacy of cancer therapies.
Stromal cells, particularly CAFs and the ECM, are critical barriers to effective drug delivery.
Patent ID: 148735 Spacial Contextualization in TME
1. Parallel Processing: Thousands of Agents, Infinite Possibilities
Consider a scenario where 10,000 people each write a unique book. For any one person to read all 10,000 books, it would take years, if not decades, and the integration of knowledge from each book would depend on that individual’s capacity to synthesize the information. In contrast, an AI system with thousands of copies of the same model can process these “books” in parallel. Each copy specializes in a different subset of the data, extracting insights, patterns, and knowledge simultaneously.
Spatial-to-Spatial AI leverages this exact principle but applies it to complex, multidimensional datasets. Imagine a network of AI agents each tasked with understanding a specific aspect of a 3-D model—whether that’s the vascular structure of a tumor or the connectivity of neuronal pathways in Alzheimer’s disease. These agents operate concurrently, focusing on different “slices” of the problem. And the beauty of it all? They share their learnings instantly.
2. Unified Language and Architecture: AI’s Advantage in Communication
Humans have evolved complex languages to communicate ideas, but there are inherent limitations. Communication is often slowed down by the need to translate thoughts into words, convey nuances, and overcome cultural or cognitive differences. Even the most sophisticated human collaborations are limited by these factors, making it difficult to fully integrate knowledge across large groups.
AI models, however, operate under a unified framework where information is shared as gradients and parameters—a common “language” built into their architecture. In the context of Spatial-to-Spatial AI, this means that each agent’s findings are not abstract interpretations but precise updates that the entire network can immediately integrate. Thousands of AI agents “speak” this language fluently, allowing them to merge their discoveries in real time and improve their collective understanding.
For instance, when AI agents map the progression of a neurodegenerative disease like Alzheimer’s, one agent might focus on vascular changes, while another concentrates on mitochondrial function. As they analyze different aspects, their findings are not just pooled together loosely; they are integrated into a cohesive, multidimensional model of disease progression. This level of integration and precision is something humans simply cannot achieve.
3. Spatial-to-Spatial AI: Revolutionizing Precision Medicine
Spatial-to-Spatial AI’s ability to integrate vast, multidimensional data is its most significant strength. Traditional AI models typically analyze 2-D data (e.g., MRI scans or histological slides). While helpful, these approaches overlook the complexities of spatial interactions, such as how a tumor’s microenvironment evolves in three dimensions or how various metabolic and vascular pathways interact in the brain.
Spatial-to-Spatial AI breaks through this limitation by processing data in its natural 3-D state, enabling a detailed and accurate understanding of complex biological systems. Here’s how:
-
Cancer Research: AI agents work collaboratively to model the tumor and its microenvironment as an interconnected system. Some agents focus on understanding how cancer cells migrate, others on immune cell infiltration, and yet others on the extracellular matrix. By pooling these insights, the AI creates a hyper-detailed spatial map that reveals the full extent of the tumor’s growth patterns and immune response. This facilitates the development of precision immunotherapies tailored to target the exact characteristics of an individual’s tumor.
-
Alzheimer’s Disease: Spatial-to-Spatial AI agents analyze neurovascular structures, metabolic activity, and mitochondrial dysfunction in a holistic manner. Each agent specializes in a different aspect of the brain’s pathology. When their findings are combined, the AI produces a dynamic, 3-D model that can predict disease progression with unprecedented accuracy, enabling early interventions that are precisely targeted to the individual’s unique disease trajectory.
-
Spatial Map Of Tumor Heterogeneity
The graph shows a simulated spatial map of tumor heterogeneity. Different colors and markers represent various components:
- Red circles: Cancer cells, indicating the primary tumor mass.
- Green circles: Immune cells infiltrating the tumor microenvironment.
- Blue squares: Blood vessels, highlighting the vascular network within the tumor.
- Purple triangles: Hypoxic regions, areas with low oxygen levels that may affect treatment response.
This spatial map illustrates the complexity of tumors, emphasizing the interactions between different cells and structures within the microenvironment. This kind of visualization helps researchers understand tumor behavior and plan targeted interventions.
4. Beyond Human Capacity: Precision and Efficiency
The ability of AI agents to operate in parallel, communicate fluently, and integrate information with mathematical precision is where the system’s true potential lies. Human specialists, despite their expertise, can only handle a limited amount of information at any given time. They rely on years of training, reading, and collaborative work to develop a comprehensive understanding of complex issues. Even then, the information they absorb is only as accurate as their interpretation and synthesis allow.
In contrast, Spatial-to-Spatial AI rapidly processes and integrates vast amounts of data across multiple domains, reaching levels of precision and efficiency that humans cannot match. For example, in developing cancer immunotherapies, the AI doesn’t just suggest broad treatment strategies; it designs hyper-personalized interventions based on the precise molecular and spatial configuration of the tumor, down to the behavior of individual cells and their microenvironment.
5. The Implications for Future Medicine
Spatial-to-Spatial AI represents a new frontier in precision medicine. By mapping and understanding complex biological systems in their full spatial and temporal dimensions, this technology enables a proactive approach to disease management. AI’s ability to share data seamlessly and operate across parallel agents opens the door to interventions that are not just reactive but predictive.
For Alzheimer's disease, this could mean pinpointing and addressing metabolic and neurovascular dysfunctions before symptoms even appear, significantly altering the disease’s trajectory. For cancer, it could mean real-time adaptation of treatments as the tumor evolves, ensuring that immunotherapies are always one step ahead of the disease.
Conclusion: The Future of Collaboration Lies in AI
The traditional human model of learning and collaboration, while effective for many domains, is limited by time, language, and individual capacity. AI, particularly Spatial-to-Spatial AI, transcends these limitations. Thousands of AI agents can work together simultaneously, sharing precise data instantly and integrating it into complex models that push the boundaries of our understanding. In fields like precision medicine, where complexity is the norm, this capability is not just advantageous—it’s revolutionary.
As we move forward, AI’s ability to collaborate in ways beyond human capacity will drive breakthroughs in the understanding and treatment of diseases that have long eluded us. The era of Spatial-to-Spatial AI has arrived, and with it comes the promise of a future where the integration of knowledge happens at a speed and scale never before imagined.
Combining Liquid Biopsy with Spatial AI... Precision Immunotherapy
- Dynamic Monitoring: Unlike tissue biopsies, which provide static snapshots, liquid biopsies offer continuous and real-time monitoring of cancer progression and treatment response. Integrating this with spatial data allows for adaptive and more personalized treatment strategies.
- Precision in Tumor Microenvironment Analysis: Spatial AI, when linked with liquid biopsy data, can enhance our understanding of the tumor microenvironment (TME). For example, it can reveal how immune cells interact with the tumor and how those interactions change over time, which is vital for improving precision immunotherapy.
- Early Detection and Prediction: By combining genetic and epigenetic markers from liquid biopsies with imaging data, AI models can predict tumor evolution and metastasis risk. This could lead to earlier detection of micro-metastases and better management of cancer before it spreads.
- Mapping Tumor Evolution and Resistance Mechanisms: LFMs can track genetic mutations and resistance mechanisms emerging over time through cfDNA analysis and correlate these with spatial changes in tumor structure, providing critical insights into therapy resistance.
AI and precision immunotherapy
"As a radiologist, I truly understand the struggles of precisely mapping a tumor, no matter where in the body it is, depending on its growth pattern. Since I work with breast cancer, many times I see a cancer growing diffusely into the surrounding tissue, and we have to give an ‘estimate’ of the size, regardless what imaging modality we use.
Unfortunately I also experience how ‘stubborn’ the medical world can be and how hesitant medical professionals are in regards to using AI in their clinical work."
"It's insightful to hear this perspective, especially from a radiologist working directly with breast cancer. The challenge of accurately mapping tumors, particularly those that grow diffusely into surrounding tissue, highlights a key issue in oncology—one that AI and advanced imaging technologies could help address. The 'estimate' approach you mentioned is a reminder of the limitations of current modalities, which often don't provide a full, precise picture of the tumor and its microenvironment.
Your observation about the hesitancy in the medical community to integrate AI into clinical practice is also crucial. It's understandable, given the cautious nature of medicine and the potential risks involved with new technologies. However, this is precisely where education and collaboration between AI developers and healthcare professionals become essential. Bridging that gap and demonstrating how AI can enhance, rather than replace, clinical expertise could foster greater acceptance and trust.
AI, particularly Spatial AI, holds promise in offering more precise and three-dimensional insights into tumor behavior, potentially moving beyond the limitations of traditional imaging. By combining radiology expertise with AI, there’s an opportunity to create models that understand and map tumor growth with unprecedented accuracy, eventually making the 'estimate' more of a precise calculation."
Clinical Trial Simulations
- Simulated clinical trials using Digital Cousins before moving to real-world trials. Insights from these trials can inform the Digital Twin about which treatments to prioritize for a specific patient. By incorporating real-world evidence from trials, the Digital Twin can continuously improve its predictive accuracy.
Patent ID-148725
System and Method for Ethics and Bias Management in Cancer Treatment Using Digital Cousins, Digital Twins, and Spatial-to-Spatial AI
Patent ID-148720
System and Method for Adaptive Treatment Pathway Optimization Using Spatial-to-Spatial AI, Digital Cousins, and Digital Twins
Patent ID-148716
System and Method for Predictive Personalization Across the Cancer Lifecycle Using Spatial-to-Spatial AI and Digital Twins
Patent ID-148715
System and Method for Real-Time Cross-Communication and Adaptation Between Digital Cousins, Digital Twins, and Spatial-to-Spatial AI for Precision Cancer Treatment
Patent ID-148714
System and Method for a Unified Data Ecosystem for AI-Driven Cancer Treatment Using Digital Twins, Digital Cousins, and Spatial-to-Spatial AI
Patent ID-148713
System for Predictive Prevention of Tumor Progression and Drug Resistance Using Spatial-to-Spatial AI and Digital Twins
Patent ID-148710
System and Method for Accelerating Drug Development, Personalized Dynamic Treatment, and Predictive Prevention Using Digital Twins, Digital Cousins, and Spatial-to-Spatial AI
Patent ID-148717
System and Method for Simulated Clinical Trials Using Digital Cousins to Enhance Treatment Personalization in Digital Twins
Neuroteg AI
Kasterlee
Belgium
Email: info@neuroteg.com guy@neuroteg.com
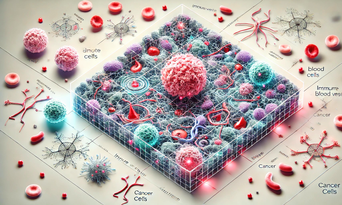
2D TME The same in 3D
Enhanced 3D representation of a tumor microenvironment (TME), designed to be highly detailed and immersive. It features intricate spatial arrangements, dynamic cellular interactions, and realistic visual effects to showcase the potential of 3D spatial intelligence in biomedical research. This version captures a richer and more interactive view for exploring complex environments like the TME.
If we start from a MRI scan in 3D
3D TME map, which shows spatially distinct regions with immune cells, tumor cells, and blood vessels, several immunotherapy strategies could be particularly effective:
-
Checkpoint Inhibitors to Facilitate Immune Cell Access:
- Objective: If the map reveals that immune cells are clustered around blood vessels but struggle to penetrate the tumor core, a checkpoint inhibitor like anti-PD-1 or anti-PD-L1 therapy could be helpful. These inhibitors target pathways that cancer cells use to evade immune detection, thus allowing immune cells to enter deeper into the tumor mass. By blocking immune suppression in identified immune-accessible zones, these therapies could enhance the immune system’s ability to reach and attack cancer cells more effectively.
-
CAR-T Cell Therapy with Navigation Enhancements:
- Objective: In regions where immune cells need to migrate across inhibitory zones to reach the tumor, CAR-T cells engineered to overcome specific immune checkpoints or physical barriers could be valuable. For example, CAR-T cells could be modified with receptors that help them target specific ligands or signals associated with tumor cells, ensuring they follow the most efficient pathway highlighted on the map toward the tumor core.
-
Vascular Normalization Therapy for Improved Infiltration:
- Objective: If blood vessels surround but do not penetrate the tumor core, anti-angiogenic agents (like bevacizumab) could be administered to "normalize" the abnormal tumor vasculature. This approach enhances blood flow, reducing hypoxia (low oxygen areas) and making it easier for immune cells to infiltrate the tumor. Vascular normalization could be paired with other therapies, such as checkpoint inhibitors, to allow immune cells better access along the vascular pathways seen on the map.
-
Combining Immune Activators with Tumor-Penetrating Peptides:
- Objective: If immune cells are detected in areas near the tumor periphery but are unable to reach the tumor cells effectively, immune-activating cytokines (like IL-2 or GM-CSF) combined with tumor-penetrating peptides could promote immune infiltration. Tumor-penetrating peptides can aid immune cells in crossing the TME’s dense extracellular matrix, allowing immune cells to reach the tumor more effectively when activated.
-
Localized Radiation or Phototherapy for Immune Priming:
- Objective: Targeted radiation or phototherapy can be used to "prime" specific tumor regions, making cancer cells more vulnerable to immune attack by upregulating immune targets. This approach could be particularly useful in areas marked as tumor-dense regions, creating an environment more favorable to immune cell activation and facilitating immune recruitment.